For Part 1, looking at reconciling the reports for diagnosis, go here.
Thanks to the generosity of #dedoc°, I recently had the privilege of virtually attending the world’s largest Diabetes conference: EASD 2021. Arguably the biggest news at the conference was an international consensus on the diagnosis, treatment, and management of Type 1 Diabetes. Interestingly, last year an international consensus was released for the diagnosis, treatment, and management of LADA. In Part 1 I reviewed how the two differed in terms of the diagnosis of Type 1 and LADA. In this second and final part I will look at the two reports’ recommendations for treatment and consider questions such as:
- Should someone diagnosed with LADA go onto insulin immediately?
- Are there treatments for Type 1 other than insulin?
- If I do use insulin what are the pros and cons of the various methods of delivery?
As usual, for those who want the short version, you can go to the tl;dr section at the end.
Where We Landed In Part 1
In Part 1, I concluded the diagnosis flow chart from the Type 1 report was the more detailed and effectively covered LADAs flow chart.
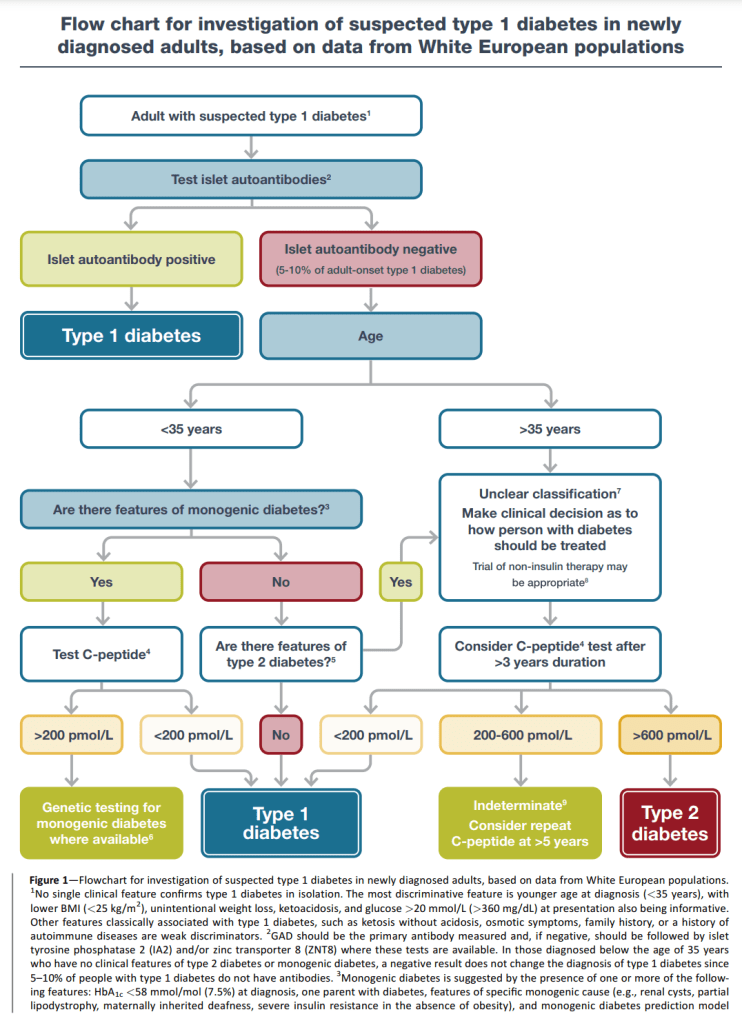

So, assuming someone has LADA or Type 1 diabetes means either:
- We have some reason to suspect diabetes (unintentional weight loss, ketoacidosis, glucose > 20 mmol/L (>360 mg/DL) etc.)
AND
- Auto-antibody presence OR
- Low C-peptide (less than 200 pmol/L (0.2 nmol/L) ) OR
- No features of Type 2 diabetes (BMI >= 25 kg/m^2, no weight loss, no ketoacidosis, less severe hyperglycaemia etc.)
Treatment According to the LADA Report
The LADA report has a flow chart for treatment which looks like this:

The Type 1 C-peptide limit is different (0.2 nmol/L vs 0.3 nmol/L) but, given there are two other options available which do not consider the C-peptide level in the Type 1 report (auto-antibody presence and no Type 2 features), there is still the possibility that someone with Type 1 could have a C-peptide in any of the above three ranges.
I go through the LADA and Type 2 guidelines in detail in my “Gold Standard” LADA article. In short, if your C-peptide is over 0.7nmol/L (700 pmol/L) options include:
- Metformin
- GLP-1 RA
- SGLT-2i
- DPP-4i
- Basal insulin
- TZD
While part of the Type 2 algorithm, there is a notable exception of Sulfonylureas not being used with LADAs because “The panel concluded that sulfonylureas are not recommended for the treatment of LADA, as deterioration of b-cell function as a consequence of this treatment cannot be ruled out”.
For patients with a C-peptide below 0.7 nmol/L, there are two flow charts. The first is if heart (ASCVD/HF) or kidney (CKD) disease is present with the same medications as before except TZDs which may have been excluded because of the limited evidence of benefit and increased risk of bone fracture.

For patients without heart or kidney disease, we have this chart where the SUs are still not present but which does include TZDs.

What is good is this set of flow charts covers the entire Type 1 C-peptide spectrum which means, even when someone with LADA becomes a “classic” Type 1 because of declining C-peptide levels, we have a prescribed course of action. What is missing is a complete answer to the question “When should someone with LADA start using insulin?” The answer from the above flow charts is “If the HbA1c is above target” but no target is firmly established. Let us move to the Type 1 report.
Treatment According to the Type 1 Report
In fact, the Type 1 report immediately addresses the issue of targets for Type 1 in their first table.
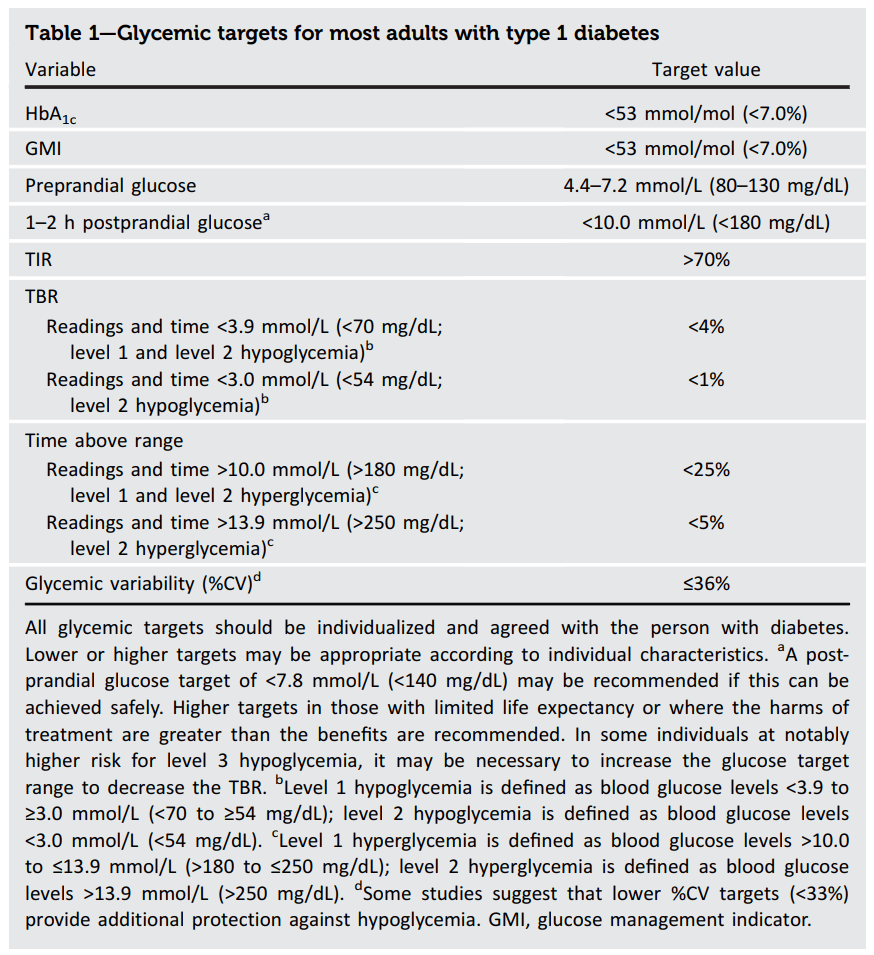
Here the target HbA1c is 7.0% with the caveat that “all glycemic targets should be individualized and agreed with the person with diabetes.” So, unless we have discussed and agreed on a different target with our health care team, achieving an HbA1c equal to or below 7.0% is a good benchmark for considering moving to the use of insulin. This is in agreement in my post where I considered how high someone’s HbA1c could be before a significant risk of long term damage.
For the specific question of when someone with LADA should consider bolus insulin, we also have guidelines for post-prandial (after meal) insulin levels with the suggestion that 1-2 hours after a meal a person’s glucose level should be less than 10 mmol/L (180 mg/dL) and the option of pushing this to less than 7.8 mmol/L (140 mg/dL) if safe to do so.
In contrast to the LADA report, the Type 1 report takes an “insulin-first” approach saying “The cornerstone of type 1 diabetes
therapy is insulin replacement” and providing the following summary of the multi-pronged approach suggested for the newly diagnosed.

Given how difficult it can be to manage insulin therapy in the newly diagnosed, it acknowledges the need to prepare for hyperglycemia (“highs”) and hypoglycemia (“lows”).
The Type 1 report also talks about the relative merits for the different ways of delivering insulin.

Where money is no object, clearly, closed-loop technology is the winner.
Eventually (page 27 out of 37 pages), the Type 1 report talks about “Adjunctive therapies”. In other words, treatments which can be used alongside insulin.

There is common ground between the two reports with both reports mentioning Metformin, GLP-1 RA, and SGLT-2i. It also mentions pramlintide which is an amylin analogue (another hormone produced by the beta cells and, therefore, compromised in Type 1 diabetes). It fails to mention DPP4i and TZD. TZD may be because of the limited evidence but I am not sure why DDP4i’s were left off the list. They affect the same hormone cycle as GLP-1 RAs and therefore have similar effects/benefits.
Reconciling the Two Reports
In contrast to Part 1 where I sided with the Type 1 flow chart for diagnosis, here I am siding with the LADA report for treatment. There are a few reasons for this:
- It explicitly considers treatment in the presence of heart and kidney disease
- It offers a more comprehensive range of non-insulin treatment options e.g. DPP4i and TZD (but should likely include Pramlintide as well)
- It takes the approach that insulin may not be necessary in patients with high C-peptide levels and, given the inherent hypo/hyper risk that comes with using insulin, if target ranges can be maintained, this seems like a sensible approach to me
This being said, the Type 1 report is much more comprehensive in considering the various ways of delivering insulin to the body (injection, pumps etc.) and also has a lot to say about looking beyond medication for individualised treatment e.g. considering lifestyle factors and diabetes education.
One big takeaway for all people with Type 1 or LADA should be that treatment no longer begins and ends with insulin. There are a range of other medications which can help with managing long term blood glucose levels and have other benefits such as helping a patient lose weight or reduce blood pressure.
tl;dr
Arguably, the LADA report’s flow charts for the treatment of Type 1 diabetes are more detailed for treatment than what is presented in the Type 1 report. Not only, does the LADA report consider insulin independence for patients with high C-peptide levels, it considers which medications are appropriate in the presence of heart or kidney disease. However, the Type 1 report fills in a significant gap of providing target values to chase and which help inform decisions such as when to move to insulin therapy.
The Type 1 report also goes into more detail in the areas of:
- The relative merits and costs of different insulin delivery methods
- Treatment of Type 1 diabetes beyond medication e.g. lifestyle factors and education
As someone with LADA, I appreciate your attention to detail on this topic immensely. I am myself hesitant to go on prescription medications so I am focused primarily on “input management” – i.e., reducing glucose through reduction of carbohydrate consumption.
One thing missing from your review I feel is, at least in the U.S., there is also an inhalable insulin method (Afrezza) that is quite compelling for those who prefer rapid, short-lasting mealtime adjustments more than anything else to remain in range.
LikeLike
If you can maintain your targets through dietary management alone that is great. I adopt an approach of reducing carb intake to minimise glucose fluctuation as well but I do use non-insulin medications as another tool in the kitbag to prolong the insulin independent honeymoon.
In terms of Afrezza, you’re quite right, the Type 1 Report, from memory, did not consider inhalable insulins. This is a shame because it has a lot of potential. I also hear the rapid onset of Afrezza has an unusual effect of protecting from hypos i.e. it is hard to inhale too much. If this is the case it would make for compelling advantage over injectable insulin.
LikeLike
Are you comfortable sharing what medications you’re using to prolong your honeymoon?
I actually take Afrezza (instead of medications), but I am considering medications if they actually act to prolong the life of my beta cells.
LikeLike
Such a great report! Helped me get insight in the complexity of the different types! Thanks so much, will recommend to friends to read this! 😉 👍
LikeLike
Hi Jon,
It seems WordPress is limiting me to just two replies deep.
I take Metformin because I have insulin resistance and this reduces the resistance and resultant stress on the pancreas and also Ozempic which is a GLP-1 RA. It promotes satiety and also helps the body release insulin quicker at meals so the insulin starts acting before the BGLs get too high. While insulin promoters like Sulfonylureas have been shown to burn out the pancreas, GLP-1s seem to have the opposite effect possibly because they also turn off automatic cell death (apoptosis) of beta cells. I wrote about incretin mimetics (which GLP-1 RAs belong) here: https://practicaldiabetic.com/2020/10/10/the-benefits-of-incretin-mimetics-for-all-diabetics/
Leon
LikeLike